Computational and Cognitive Neuroscience
The considerable and rising economic burden of neurological, psychiatric and cognitive disorders, mainly due to an absence of effective treatments along with an increasingly elderly population, poses an urgent need for innovative treatments to prevent, delay onset, or alleviate symptoms of these diseases. The use of electrical deep brain stimulation (DBS) has been proven to provide striking benefits for patients with advanced Parkinson’s disease, essential tremor and dystonia who have failed conventional therapies. Moreover, promising applications of DBS for the treatment of neuropsychiatric disorders have emerged, including treatment-refractory obsessive-compulsive disorder, Tourette’s syndrome, major depressive disorder, drug addiction and anorexia nervosa. Visual neural prostheses represent an approach to restore vision in blind people by direct stimulation of the visual pathway. To date, the most advanced prosthesis intervention is retinal prosthesis. A new generation of cortical visual prostheses is currently undergoing accelerated development. We focus our research on therapeutic interventions involving neural stimulation (Deep Brain Stimulation-DBS, visual prosthesis) to treat neurodegenerative diseases and aim at the optimization of the therapeutic outcome based on modeling the pathological state neuronal activity and the effect of stimulation on tissues. Our research work includes:
- Development of multi-level hierarchical modeling approaches and efficient transition techniques between different modeling levels to study functional brain networks (basal ganglia etc.) and visual information perception networks involved in neurodegenerative diseases
- Development of biologically-inspired Computer Vision methods to improve the quality of vision restoration in visual prosthesis
- Modeling of the response of retinal or cortical neurons, utilizing various methods including deep learning methods, like convolutional neural networks and recurrent neural networks, along with model-fitting methods and appropriate datasets.
- Algorithmic design of maximally-efficient closed-loop DBS systems (for Parkinson’s disease and treatment-refractory obsessive-compulsive disorder), integrating computational models with refined optimization techniques in order to allow for the fast determination and delivery of therapeutically- and energy-efficient stimulation protocols taking into account the individual neuroanatomical variability
- Analysis of brain electrophysiological activity recorded from scalp surface or directly from brain structures aiming at gaining insights for upper cognitive functions and mental state and improving the treatment of neurological diseases through the study of intracranial sources and information flow in the brain.
Neurons and Neural Networks: Computational Models
Developing models of the Visual System – Simulating Prosthetic Vision
Visual percepts excite the retina, the outermost and initial part of the visual tract, and eventually lead to activity in the visual cortex, which is located on the occipital lobe. Aiming to restore vision, various implantable devices have been proposed, treated in theoretical experiments and finally tested in clinical trials. These devices may be implanted in different locations of the visual tract: in the retina (epiretinal, subretinal or subchoroidal implantation) or in the visual cortex. ‘Prosthetic vision’ is a term coined to describe the vision attained by the aforementioned implants.
In the framework of prosthetic vision, we focus our interest in predicting retinal and cortical response. Performing the artificial stimulation incorporating the neural code has provided very encouraging results in experimental animal studies. We apply image analysis and image enhancement methods to pre-process the input (visual information) that is provided to the models, in order to cope with the low prosthetic vision resolution or to partly implement the information processing done in the biological retina. We extract biologically inspired image features using computer vision methods based on the various Retina Ganglion Cells’ (RGC) functions. These features can then be used towards accurate models of retinal response.
Aiming at improving Retinal Prosthesis interventions, we have used RGC-inspired features in retina models, applying a standard Generalized Integrate & Fire (GIF) spike generation mechanism. In Natural Images, using the RGC-based features provided performance gains over using unprocessed images, as quantified by spike train distance metrics. Thus, the hypothesis that unprocessed images are an improper input representation for retina response prediction was strengthened by our findings.
To model the response of biological neurons, e.g. retinal or cortical neurons, linear-nonlinear models have been applied, in which an initial linear-filtering stage is followed by a nonlinear response function. Furthermore, Generalized Linear Models, in which the assumption that the neural spikes follow a Poisson temporal distribution is made, have been successfully used to model biological neural response. Integrate & Fire models (e.g. GIF), in which the input is integrated and a neural spike is generated whenever the integral surpasses a threshold, have also been applied to model the response of biological neurons. In all the aforementioned ‘traditional’ approaches, model-fitting methods (e.g. maximum likelihood estimation) may be applied on appropriate datasets.
More recently, deep learning methods, like convolutional neural networks and recurrent neural networks, have been successfully applied to model biological systems as the retina and the visual cortex. However accurate, DL are ‘black box’ models. Interpretable machine learning can be applied to elucidate the biological mechanisms, which determine properties of the visual system, from deep models. We put in focus a new generation of accurate retina and cortex models, exploring DL and traditional neuron response modeling methods, bridging the experimental procedures with computational considerations to acquire appropriate datasets of biological responses and introducing explainability to magnify the impact of the models in prosthetic vision.In retina simulations, we encode visual scenes to retinal ganglion cells’ neural spiking patterns. This neural encoding can be used in prosthetic vision to determine the optimal electrical stimulation pattern in the implant’s electrodes, i.e. to translate a visual scene to a stimulus that is delivered through the implanted device. Furthermore, in cortical visual prosthesis, the retinal encoding can be used as the input to the cortex model, in parallel to visual tract organization.
Moreover, an important topic is simulating prosthetic vision attained through the different implants in the visual tract. Critical implant limitations (electrode number and dimensions, non-selective cell stimulation, axonal stimulation, stimulus waveform), algorithmic issues (real-time functionality, modeling fidelity), anatomy issues (degenerated cells) and implant-tissue electrical interaction (area of dispersion of electrical stimuli and temporal decay), are considered to simulate the achieved prosthetic vision.
Modeling of the basal ganglia
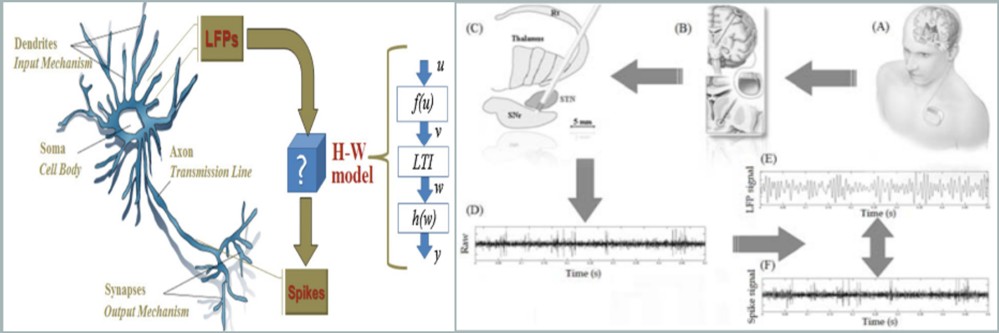
The underlying pathophysiology of movement and several neuropsychiatric disorders is almost undoubtedly attributed to the structural and functional characteristics of the basal ganglia, a group of nuclei in the center of the brain. Modeling of the brain, in general, and the basal ganglia, in specific, has begun since the early 20th century; however, the flourishing of a new therapeutic technology has rendered the entitlement with models of the basal ganglia even more challenging. Deep Brain Stimulation (DBS) has sprung from the family of lesioning therapeutic interventions twenty years ago and has ever since dominated the operating theatres for refractory types of Parkinson's disease. In the case of DBS, the approach was firstly proved to be successful in practice, while in theory, elementary concepts are still subtle and need clarification. During functional interventions and DBS electrode placing procedures, microelectrode recordings (MER) from the basal ganglia are routinely acquired. Practically, they are useful in deciding the target for the permanent placement of the DBS electrode, but their value goes way beyond that. The information included in such recordings is manifold, combining both fast spiking activity and slow activities such as local field potentials. Because of the hierarchical structure of the brain, the models can be designed to target mechanisms that belong to functional categories spanning from the subcellular to whole system levels of description.
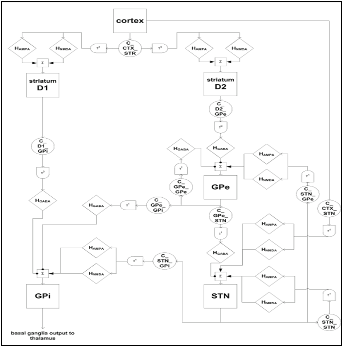
Today, it remains unclear which level of single-cell modeling is appropriate to understand the dynamics and computations carried out by such large systems. A thorough understanding of single-neuron function together with network stability and system processing effectiveness can be obtained only by relating different levels of abstraction. Trying to incorporate every biological detail of the investigated neuron is likely to obscure the focus on the essential dynamics, whereas limiting investigations to highly abstract processing schemes casts doubt on the biological relevance of specific findings. Thus, we focus on multi-level hierarchical modelling approaches and the development of efficient transition techniques between different modelling levels. Model development has been based on microelectrode recordings (MERs) acquired during DBS surgery. The study is performed via biologically plausible models that mainly follow the Hodgkin-Huxley formulation and are appropriately integrated to represent neuronal assemblies. On the system-level, various hypothetical mechanisms have been proposed for basal ganglia functioning and their participation in action selection, working memory representation, sequence production and in processing of cognitive tasks. The objective of our team, through computational models on a high level of abstraction, is to thoroughly study the major computational hypothesis characterizing the role of basal ganglia as a central switch and decision making mechanism of the brain, in both motor and cognitive tasks. Particular emphasis is given to the examination of the dopamine effects in the smooth functioning of the basal ganglia network and in the generation of characteristic Parkinsonian patterns of activity.
DBS Optimization
By simulating the neuronal activity in the pathological state and the effect of stimulation on this activity, we aspire to develop sophisticated techniques for the optimization of the therapeutic outcome of DBS.
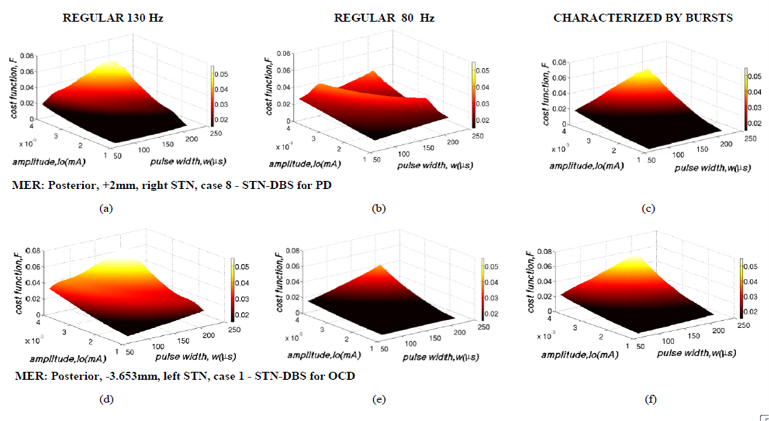
Central to our team's efforts is to combine modeling approaches with real intra-operative microelectrode recordings (acquired by the Neurosurgical Clinic of the University of Athens). Also, since modeling of the basal ganglia together with DBS data and control can supply information about the underlying facts, the models of the basal ganglia are designed by our group in a way that can support simulations of the DBS interaction with the tissues, thus providing indications for the validity of the approach. Other goals include the determination of the functional characteristics parameters as well as the exact placement of the brain stimulating electrode, during the DBS intervention. We have focused our efforts on the algorithmic design of a maximally-efficient closed-loop DBS system for Parkinson’s disease and treatment-refractory obsessive-compulsive disorder. The control system aims at integrating the developed computational models with refined optimization techniques in order to allow for the fast determination and delivery of therapeutically- and energy-efficient stimulation protocols. Delivery of stimulation is adjusted to the fast dynamics of movement and neuropsychiatric disorder symptoms through the reliable assessment of robust biomarkers that capture the patient’s clinical state in ‘real-time’. Apart from substantially improving the therapeutic outcome, application of these systems in clinical practice aims at the gradual replacement of post-operative clinical management, which is a considerably time consuming and not optimally efficient procedure. Moreover,it aims at minimizing energy consumption and reducing the frequency of generator replacement surgery, the concomitant risk of hardware infection or the rate of recharging procedures.
Analysis of Brain's Electrophysiological Activity
Feature extraction and classification
Various features are extracted from brain electrical activity recordings -using autoregressive and multivariate autoregressive models, harmonic analysis, wavelet transformation, energy of the characteristic frequency rhythms (α,β,δ,θ) in order to reduce dimensionality and to focus on specific signal characteristics. Using these features, biosignals are classified to normal and abnormal patterns, while internal brain structures are identified from their signal signature, by means of intelligent computational methods. Moreover, research efforts of our team refer to the integration of novel mathematical methods with results from psychoacoustic analyses and recorded EEG and ERP signals in order to provide insights into the various mechanisms participating in the perception and processing of acoustic information. Our interest focuses on the assessment of potential alternations in EEG and ERP recordings, during acoustic stimuli, due to exposure to electromagnetic radiation (EM) of third generation mobile terminal. An experimental adult volunteers' study has been designed and carried out. Numerical assessment of the EM power absorbed by functional brain structures is carried using a specially designed numerical dosimetry tool for brain substructures, based on the Talairach-Tournoux brain Atlas. The study is concluded with the statistical analysis of EEG and ERP recordings and signal processing in the time domain, using innovative methods and emphasizing on specific frequency bands of brain activity.
Seizure prediction
Epilepsy afflicts about 1% of the world's population, or more than 50 million people worldwide. A most disabling characteristic of epilepsy is the unforeseen way seizures occur. Algorithms with high sensitivity and specificity, leading to an early prediction of an upcoming seizure, need to be designed to "drive" "intelligent" implantable or non-implantable devices to electrically stimulate, or, alternatively, infuse drugs to appropriately chosen regions of the brain to avert the seizure occurrence. Alternatively, such devices could just inform the patient about the upcoming seizure so that he could take the necessary actions to avoid injury.
Various studies have come up with evidence for the existence of a pre-ictal period that could be detected using various characteristic measures. The ultimate goal of our group's work is to achieve early prediction of epileptic seizures in a patient-specificmanner with high sensitivity and specificity, based on surface EEG (sEEG) recordings of the patients. The data used in our study are acquired in the Long-Term Video - EEG Monitoring Unit of the Epilepsy Surgery Unit at "Evangelismos" Hospital in Athens, Greece, during pre-surgery evaluation of patients with refractory Temporal Lobe Epilepsy (TLE), who are candidates for epilepsy surgery. Various algorithms towards seizure prediction are investigated, including univariate and bivariate measures, linear and non-linear approaches, with emphasis on the non-linear ones. These measures are derived mainly from non-linear dynamics (e.g., fractal dimension) and information theory (e.g., mutual information), while other methods, such as wavelets, are considered as well. Moreover, emphasis is given on statistical validation of the results, in order to quantify their significance and determine the specificity and sensitivity achieved by the methodologies. Unlike most of the published studies which focus on the use of intracranial EEG data (obtained with depth electrodes), our efforts are focused on sEEG data, since the latter can be used in a non-invasive approach. Such an approach is definitely more attractive and can lead to a safer and practical seizure prediction and abortion protocol. Towards that direction, additional methodologies for automated artifact detection or suppression are also investigated.
Brain connectivity patterns
Rhythmicity is a useful characteristic examined in brain electrical activity recordings because it reflects the synchronization of neuronal activity, which in turn reflects changes of neuronal membrane potential. If interdependence of temporal relations of brain rhythms does exist, it is possible that rhythms observed in various brain regions are reciprocally related, and this rhythmicity is important for the integration of mental processes. The degree of synchronization between brain structures is analyzed by coherence techniques revealing the causal relation (direction of influence) between electrophysiological signals. It is considered that propagation of brain activity is related to the information flow and cortical connectivity of human brain and can be understood as the causal influence of one structure on the other.
Methods for the estimation of the causal influence are developed for both direct and indirect interactions among EEG/ERP signals. The findings can be visualized in brain maps showing the connectivity among brain structures. The study of information flow can provide insights for upper cognitive functions and mental state and it is examined for understanding neurophysiological disorders in obsessive compulsive disorder, schizophrenia and dyslexia. This information is also important in the treatment of neurological diseases, because it can reveal the spatial propagation of an epileptic seizure along time or how brain structures in motor cortex communicate to each other in Parkinson's disease.
Intracranial source localization
Determination of intracranial sources of brain electrical activity based on EEG/ERP surface recordings, is of great importance since it allows to improve our understanding of the basic mechanisms of cognitive processes and a better characterization of pathologies. To this end, various techniques are used that follow different approaches and assumptions. The choice of the most suitable technique is better-aimed if there is a priori information on the sources and the shape of head. In addition, methods for the integration of source imaging results with anatomical data are developed, to define the coordinates of the active sources in terms of Broadmann areas or in Talairach coordinates and to draw conclusions about the anatomical/functional structures being active. Clinical applications of noninvasive source imaging include improved understanding and diagnosis of disorders such as depression, schizophrenia, Parkinson's and Alzheimer's disease. Moreover, source localization combined with personalized imaging data constitutes a powerful tool for neurotherapeutic tasks, such as surgery-based therapy in Epilepsy.
